We are happy to announce a new paper from our Team, focusing on two interesting proteins – human Kti12 and PSTK.
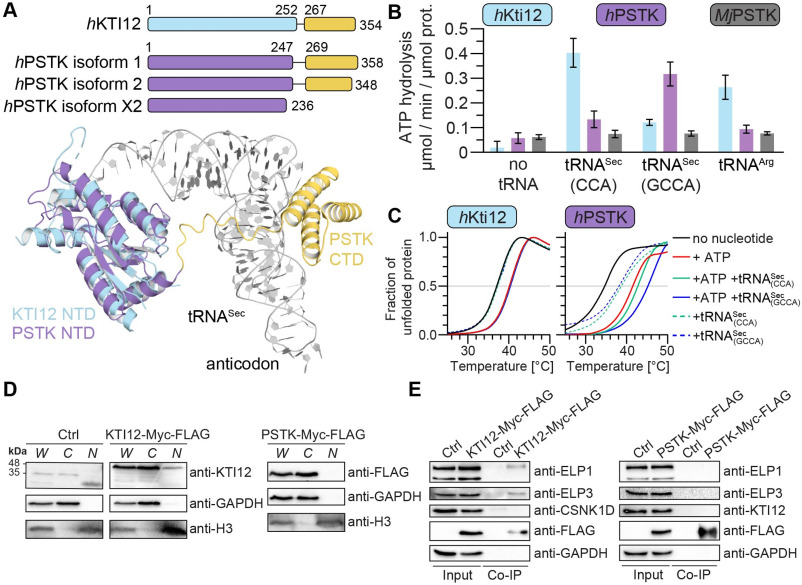
Kti12 and PSTK are highly similar proteins implicated in different aspects of tRNA metabolism. Kti12 is a regulatory factor of the Elongator complex, involved in the modification of wobble uridine in eukaryotic tRNAs. The mis-regulation of Elongator is associated with a variety of human diseases, including severe neurological disorders and cancer. PSTK phosphorylates the tRNASec-bound amino acid serine, which is required to synthesize selenocysteine. Kti12 and PSTK have previously been studied independently in various organisms, but only appear simultaneously in some animalia, including humans.
Our study provides the first comprehensive analysis of these proteins in human cells. We compare enzymatic activity, protein interaction network as well as localization of these proteins. We demonstrate that human KTI12 and PSTK do not share interactors or influence their respective biological functions. Our work has been published in BBA Molecular Cell Research.
You can read the publication here: https://www.sciencedirect.com/science/article/pii/S0167488920303037?via%3Dihub
Written by Marta Smejda